Delivering Therapies Where They’re Needed Most
Transforming Potential Into Progress
Every Patient Deserves a Future
Together, We Go Further
Science with Purpose
Dawn Therapeutics is a preclinical biotechnology company developing tissue-targeted delivery platforms to overcome the barriers that limit current gene-therapy approaches. Our goal is to reach Phase 1 readiness within 1–2 years for our lead programme in Hurler syndrome (MPS-I H) while advancing parallel discovery in neurological and musculoskeletal diseases.
42% Average Reduction in Toxic GAGs
Reduction in Toxic GAG Accumulation (preclinical)
Transforming Lives with Gene Therapy for Hurler Syndrome
Our patented sLV platform delivers breakthrough treatment for MPS-1H patients with unmet medical needs
- 42% average reduction in toxic GAG accumulation achieved in preclinical studies
- First therapy to address residual disease after bone marrow transplantation
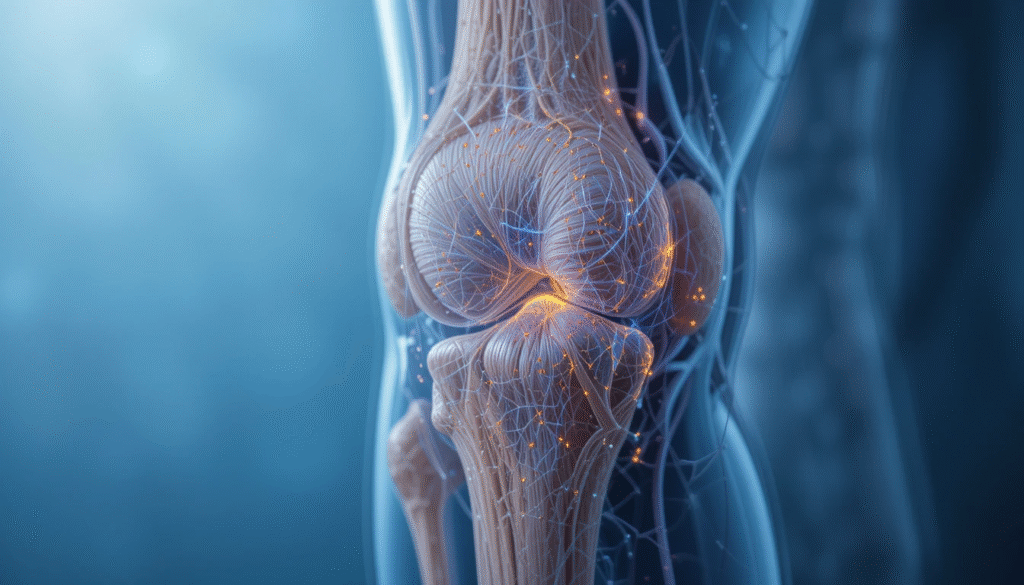
- Patented sLV technology targeting bones, joints, and cartilage
- Accelerated FDA approval pathway for a rare disease with no alternative treatments
- Potential to improve both lifespan and quality of life for affected patients
sLV Platform
Patented stealth lentiviral vector (sLV) platform engineered for targeted delivery to bones, joints and cartilage — addressing residual disease after bone marrow transplant.
Indication: MPS-1H (Hurler Syndrome)
Development status: Preclinical — demonstrated 42% average reduction in toxic GAG accumulation in target tissues.
14× Brain to Blood Ratio Efficiency
Peer-reviewed Research
Angew. Chem. Int. Ed. 2025 | doi.org/10.1002/anie.202500247
Revolutionary Brain Delivery: 14x More Effective Than Current Systems
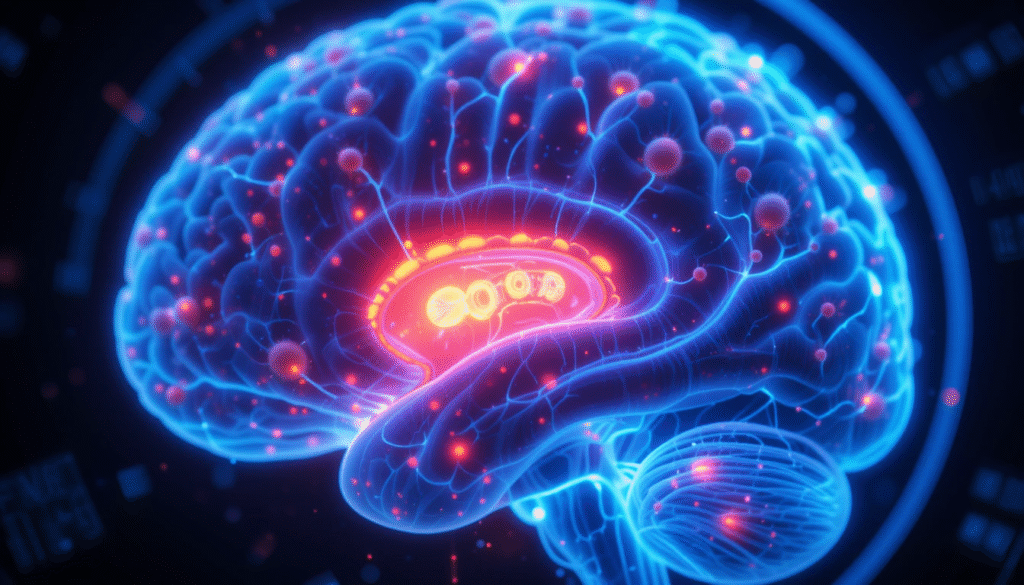
Our patented iLNP technology crosses the blood-brain barrier to treat neurological diseases
- 14-fold superior brain-to-plasma ratio compared to existing delivery systems
- Patented LNP formulation with transferrin receptor targeting
- Successfully penetrates the blood-brain barrier and reaches neurons
- Validated delivery to deep brain regions: thalamus, hippocampus, cerebellum
- Platform for treating Alzheimer's, autism, and other CNS disorders
iLNP Platform
Patented lipid nanoparticle (iLNP) formulation engineered for transferrin receptor targeting and validated deep brain delivery.
Key metric: 14× improved brain-to-plasma delivery vs current systems.
Applications: Alzheimer’s disease, autism spectrum disorders, other CNS indications.
Our Mission Statement:
- At Dawn Therapeutics, we believe the future of genetic medicine lies in precise delivery bringing DNA, RNA, and therapeutic molecules directly to the cells that need them most.
Our research focuses on two proprietary delivery platforms that can be adapted across multiple disease areas:
CNS Platform Brain-Targeting
A transferrin-receptor–guided system capable of crossing the blood–brain barrier to deliver genetic payloads to the central nervous system.
Early preclinical work has demonstrated encouraging results in Alzheimer’s disease models, and confirmatory studies are underway.
Cartilage & Bone Platform Musculoskeletal-Targeting
A delivery system engineered to reach cartilage, bone, and joint tissues for the treatment of rare genetic and acquired musculoskeletal diseases.
In preclinical models, we have successfully delivered the IDUA gene and mRNA into bone and cartilage, paving the way for future treatments of MPS-I–related skeletal diseases and conditions such as osteoarthritis and rheumatoid arthritis.
Patients & Families
We work hand in hand with patients, families, and advocacy groups to provide clear information, compassionate support, and access to cutting-edge therapies that can transform lives.
Science & Innovation
Our proprietary delivery platform enables RNA and gene therapies to reach precise target tissues, opening new possibilities for treating rare and complex diseases at their root cause.
Partnering for Impact
From early research collaborations to licensing agreements, we work with innovators worldwide to accelerate breakthroughs and bring them to patients faster.
Disease Area Tiles
Hurler Syndrome (MPS I)
A rare genetic disorder caused by enzyme deficiency, leading to progressive tissue and organ damage.
Autism Spectrum Disorder (ASD)
Alzheimer’s Disease
Addiction Disorders
Osteoarthritis (OA)
Multiple Sclerosis (MS)
Parkinson’s Disease

Mucopolysaccharidosis Type II (MPS II – Hunter Syndrome)
Rare CNS & Musculoskeletal Disorders
DTX-101 (MPS-I H)
Overview
DTX-101 is an investigational, in vivo "stealth" lentiviral vector (sLV) designed to deliver a functional IDUA gene to address Hurler syndrome (MPS-I H), a severe and life-limiting lysosomal-storage disorder.
Current Status
We have successfully achieved the pre-clinical in vivo proof of concept. Our next objective is to raise funds via investment or donation to perform GMP, Toxicology, and Phase 1 Clinical Trial.
Our aim is to progress DTX-101 to first-in-human clinical testing following successful completion of these studies and regulatory review.
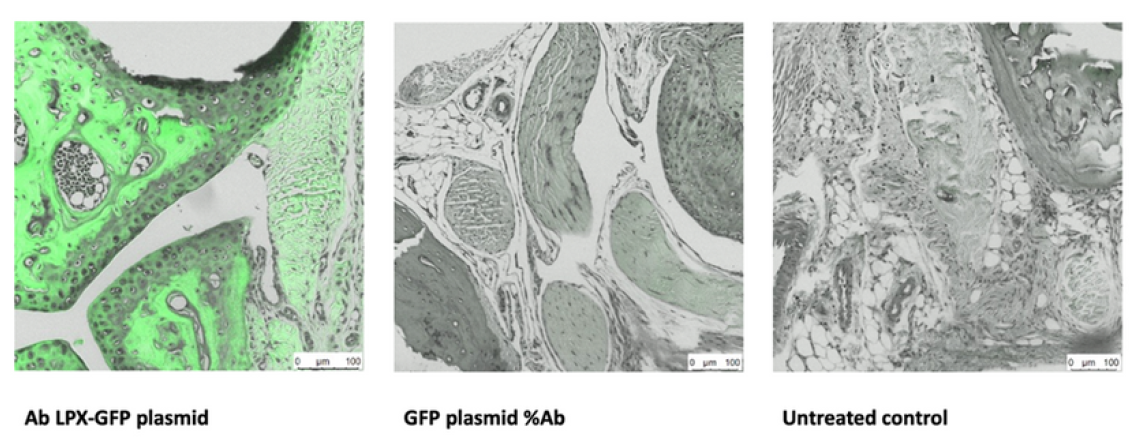
Histology: Joint sections following targeted delivery
Left: Ab LPX-GFP plasmid. Middle: GFP plasmid %Ab. Right: Untreated control. Bright green signal in treated joints confirms expression of GFP within cartilage and joint structures. Scale bars 100 µm. Leica LMS, GFP and light channels.
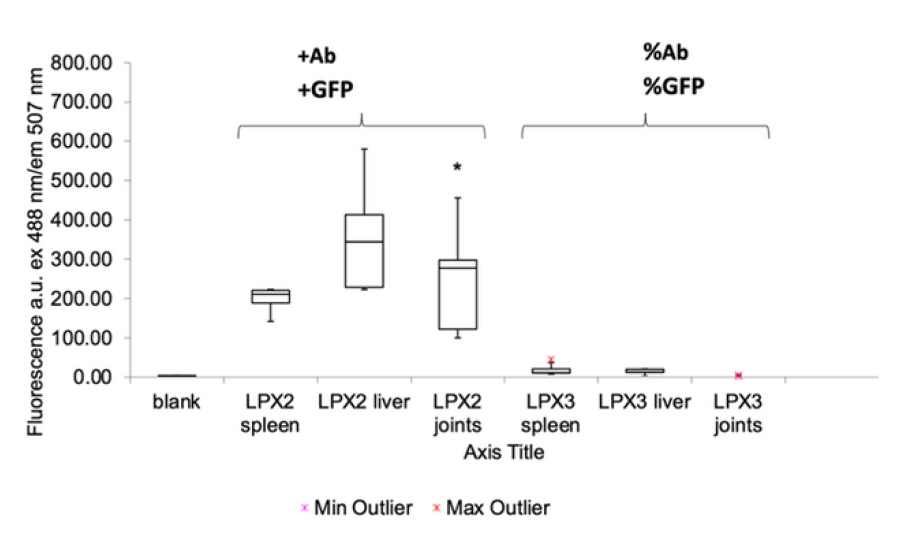
Results: GFP expression increased seven fold in joints in vivo
Box plots of GFP fluorescence (ex 488 nm, em 507 nm) demonstrate higher reporter expression in LPX2 joints versus controls. ANOVA p = 0.023 at 95 percent significance. N = 5 per group. Min and max outliers indicated.
Conclusion:
Histology shows clear signal localised to joint cartilage in treated animals, with minimal background in controls. Box plot confirms about seven-fold higher reporter expression in joints. Effect is statistically significant (ANOVA p = 0.023, N = 5 per group). Together, these data validate effective, targeted in vivo delivery & support advancement to first-in-human studies.
High-yield summary
In vivo cartilage and joint targeting was validated by histology and quantitative fluorescence readouts, supporting further development of DTX-101.
- 1Seven fold higher reporter expression in joints compared with controls. Current delivery systems achieve a brain-to-blood ratio of less than or equal to 0.5, so our delivery is 14 times more efficient. In simple terms, more of the medicine reaches the target tissue and less is wasted in the bloodstream, which can mean lower doses and fewer side effects.
- 2Robust tissue localisation in H and E sections with bright signal in treated joints. This means standard microscope stains show the signal sitting inside the joint structures where it is needed, not spread randomly, which is what you want for precise treatment.
- 3Consistent performance across animals with statistical significance at 95 percent. In practice, the results are unlikely to be due to chance and can be reproduced, which increases confidence that the effect is real.
- 4Translatable platform for IDUA and related payloads in musculoskeletal disease. The same delivery vehicle can carry the actual therapeutic gene or other cargos to cartilage and bone, helping shorten the path from lab findings to real treatments.
- 5Readouts align across modalities: qualitative histology and quantitative fluorescence. Both the pictures and the numbers tell the same story, which is a strong cross-check that the technology is doing what it should.
- 6Platform is payload-agnostic and designed to support IDUA delivery for MPS-I H. That means we can swap in different genetic instructions when needed, including IDUA for Hurler syndrome, without redesigning the whole system.
These findings provide the scientific basis to progress to GMP manufacture, formal toxicology, and first-in-human evaluation.

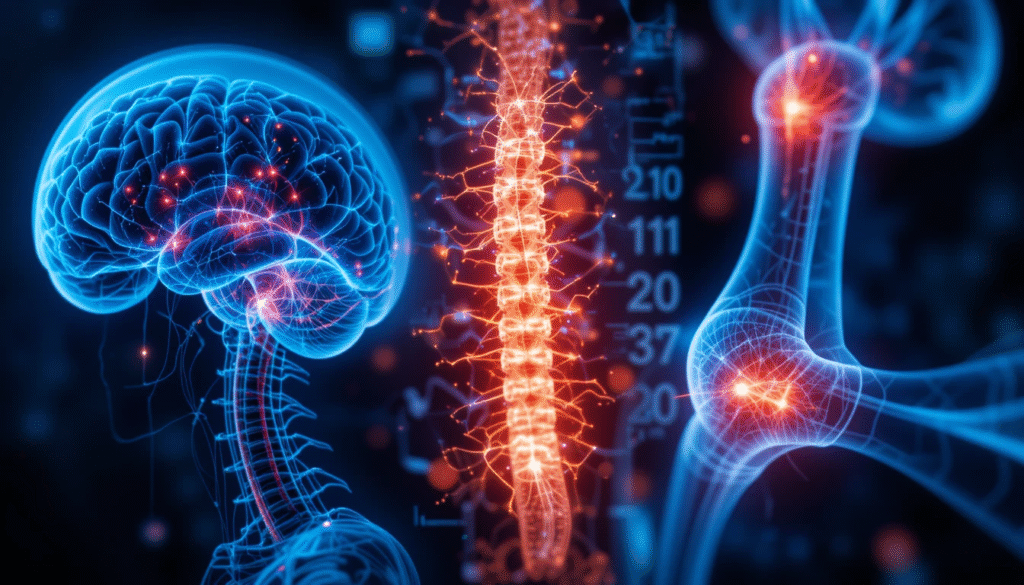
At Dawn Therapeutics, we are pioneering a new generation of targeted delivery systems that carry life-changing RNA and gene therapies directly to the tissues that need them most – from the brain to cartilage and bone. Our precision platform combines advanced vector engineering with deep
clinical insight to transform how rare and complex diseases are treated.
Precision Targeting
Advanced vector engineering delivers therapies only where they are needed, reducing off-target effects.
Payload Versatility
Carries a wide range of RNA types, gene editing tools, and small molecules.
Clinical Readiness
Built for scalability and GMP manufacturing to streamline translation to the clinic.
Programme Pipeline
DTX-101
sLV / CNS
Hurler Syndrome (MPS I)
Preclinical: Awaiting Funds
DTX-201
LNP / CNS
Autism Spectrum Disorder
In Research & Development
DTX-301
sLV / CNS
Alzheimer's Disease
In Research & Development
DTX-401
LNP / Bone
Osteoarthritis
In Research & Development
Latest News & Insights
Stay up to date with our breakthroughs, publications, and thought leadership as we work to advance targeted RNA and gene therapies.

Press Release
Dawn Therapeutics Announces New Preclinical Data for DTX-101

Publication
Targeted Lentiviral Vectors for CNS and Musculoskeletal Disorders

Conference
Dawn Presents at the Annual Gene Therapy Symposium
Science with Purpose. Commitment with Heart.
We believe that advancing breakthrough therapies goes hand in hand with building trust, fostering equity, and giving back to the communities we serve.
Advocacy Partnerships
We collaborate with patient advocacy organisations, non-profits, and research alliances to amplify patient voices and accelerate access to life-changing treatments.

Patient Stories
Through real patient stories, we celebrate resilience, share experiences, and highlight the human side of rare and complex diseases.

ESG & Responsibility
From sustainable manufacturing to diversity in clinical trials, we uphold our responsibility to the environment, society, and governance with measurable goals.
active advocacy partnerships
renewable energy in manufacturing (goal by 2030)”
patient stories shared globally
Work With Us to Advance Breakthrough Therapies
Join our team or collaborate with us to turn cutting-edge science into real-world solutions for patients worldwide
Be Part of the Future of Medicine
We are recruiting participants for our upcoming clinical trials in rare and complex diseases. Your involvement could help shape tomorrow’s treatments.

